Dietary Supplement Trade Association Projects Unprecedented Costs to Meet FDA Notification Requirement
08/31/2016 / By supplementsreport

Based on an initial assessment of new draft guidance published this month by FDA, a Utah-based trade organization projected the dietary supplement industry must devote billions of dollars to comply with a notification requirement rooted in a 1994 law.
Article by Josh Long
FDA is charged with reviewing the safety profile of new dietary ingredients (NDIs), although the agency said it has received fewer than 1,000 safety-related dietary ingredient notifications since the 1994 Dietary Supplement Health and Education Act (DSHEA) was signed into law by President Bill Clinton.
However, the United Natural Products Alliance (UNPA) estimated FDA’s new guidance could subject half or more of all dietary supplements on the U.S. market—representing tens of thousands of products—to the requirement to file an NDI notification (NDIN).
“[Our] preliminary assessment is that this will be the most expensive regulatory requirement in the history of the dietary supplement industry,” declared Loren Israelsen, president of UNPA, in a phone interview.
While the guidance does not carry the force of law, it reflects the agency’s thinking on the subject, and courts generally grant substantial deference to an agency’s interpretations of statutes governing its responsibilities. However, FDA noted firms can use a different approach if it satisfies the relevant statute and regulations.
“The FDA believes the approach to NDIs described in this draft guidance is grounded in law and necessary to protect the public health,” said Cara Welch, Ph.D., senior advisor in FDA’s Office of Dietary Supplement Programs (ODS), in an emailed statement.
The law exempted old dietary ingredients (ODIs)—those marketed before Oct. 15, 1994—from required NDI submissions. However, Israelsen expressed concerns that FDA’s 2016 guidance broadly interprets the list of substances on the market today that would meet the definition of an NDI and require a submission to FDA. For example, Israelsen described as “excessive” FDA’s list of manufacturing changes that would transform an old ingredient into an NDI, and he asserted the list “does not address or change the public health and safety equation.”
During an August 25 webinar for UNPA members to analyze the new guidance, Israelsen referenced FDA’s position that a combination of two NDIs in a product makes the finished product an NDI. UNPA is concerned that such a proclamation could be interpreted by FDA as requiring a separate notification for a dietary supplement containing the two ingredients, even if FDA previously acknowledged receiving NDI notifications for each of the two dietary ingredients.
“What FDA … is trying to tell us is that ‘We view the world as new,’” Israelsen said. “’Everything you sell is new unless you can demonstrate by exemption or exception that you’re old.’”
As part of its analysis, UNPA has circulated for comment a document outlining the projected economic impact of the NDI guidance on the industry, ranging from US$1.9 billion to $5.7 billion, based on FDA-supplied figures estimating the number of dietary supplement products on the market in 2012 (55,600) and annual product introductions (5,560). UNPA’s economic estimate is based on a projection that 50 percent, or half, of the finished dietary supplement products presently on the market would require an NDI notification.
UNPA’s analysis included a number of assumptions and took into account FDA’s definitions in the new guidance, such as its interpretation of manufacturing changes and chemical alterations that would also transform a number of ODIs into a new substance requiring an NDIN. (DSHEA specified the notification requirement doesn’t apply to a dietary supplement that “contains only dietary ingredients which have been present in the food supply as an article used for food in a form in which the food has not been chemically altered”; however, FDA’s 2016 guidance cited examples of processes that could chemically alter an ingredient, consequently rendering inapplicable the above exemption).
The estimated costs of compliance also are based on the projected average costs of an NDI submission. Assuming the average cost of an NDI notification is $50,000, UNPA forecasted $1.9 billion in economic costs to industry. The projected economic costs skyrocket to nearly $5.7 billion, presuming the average cost of an NDI notification is $150,000, according to UNPA’s analysis.
“Based on our initial analysis of the revised guidance as well as the numbers supplied by FDA, when you include the agency’s proposed definition of what constitutes a manufacturing change, the broad reading of chemical alterations and non-qualifying dietary ingredients, the removal of synthetic botanicals and what appears to be a shrinking ODI list in favor of an NDI-based regulatory structure, we think that the number of products that would require an NDI notification is conservative and reasonable,” said Frank Lampe, UNPA’s vice president of communications and industry relations, in an emailed statement.
“As part of our due diligence on the cost implications of the new guidance, UNPA is huddling with its NDI/GRAS Working Group to try to further dial in NDI notification costs,” Lampe added. (GRAS, standing for generally recognized as safe, is a safety standard that applies to conventional foods. In the 2016 NDI guidance, FDA cited certain conditions in which an NDI notification is not required if a new dietary ingredient has been affirmed by FDA as GRAS).
FDA previously estimated that it will take no more than 20 hours to research and generate new safety data for an NDI submission. “We believe that the burden of the premarket notification requirement on industry is limited and reasonable because we are requesting only safety and identity information that the manufacturer or distributor should already have developed to satisfy itself that a dietary supplement containing a new dietary ingredient is in compliance with the FD&C [Federal Food, Drug & Cosmetic] Act,” FDA stated in a 2015 document.
Under the law, a product containing an NDI is considered “adulterated” unless there is evidence demonstrating the safety of the ingredient. The above provision of the law is separate from the premarket notification requirement, the agency pointed out.
In 2015 comments filed with FDA, the Alliance for Natural Health USA (ANH-USA) argued the agency “grossly underestimated the burden of the NDI notification process on the dietary supplement industry.”
FDA is “attempting to artificially separate the work that goes into manufacturing or distributing new dietary ingredients or dietary supplements that contain an NDI,” Gretchen DuBeau, executive and legal director of ANH-USA, wrote in Jan. 13, 2015 comments. “The evidence of safety and premarket notification requirements … are inextricably combined.”
Based on research of its own member companies, the Natural Products Association (NPA) revealed toxicology studies cost on average between $178,000 and $328,000. NPA pegged at $162,500 the average cost of full-time employees and consultants for preparing the NDI submission.
Daniel Fabricant, Ph.D., executive director and CEO of NPA, said NPA members have invested on average between $350,000 and $500,000 on a successful NDI submission.
George Burdock, Ph.D., president of the safety and regulatory consulting firm Burdock Group, said in an emailed statement that it would be difficult to predict the costs of safety studies in support of an NDI filing.
FDA’s draft guidance, Burdock explained, “describes some very subjective qualifiers [that] can influence the number and type of safety studies required.”
However, Burdock said the costs for tests alone could exceed $1.5 million to demonstrate the safety of a new ingredient depending on the circumstances. He referenced various studies such as chronic toxicity, teratology, and ADME (absorption, distribution, metabolism and excretion) studies.
“If ODS [FDA’s Office of Dietary Supplements] agrees with the notifier that there has already been considerable exposure to a dietary supplement, the test burden could be quite low and relatively inexpensive,” he noted. “However, if there has been very little exposure (or exposure could not be documented) and/or the exposure in a dietary supplement will be significantly more than historical use and, especially if the directions for use indicate chronic consumption, the test burden will be very high.”
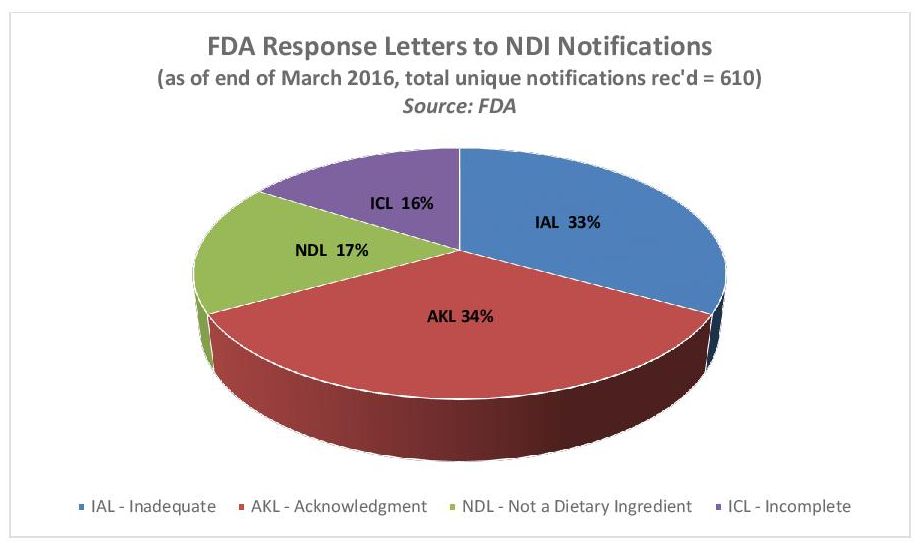
As INSIDER reported in January, FDA has objected to the majority of NDI submissions for various reasons, such as a finding that the submission wasn’t complete enough to satisfy the regulation’s requirements or the safety data was inadequate (see chart below).
Fabricant described as “far-fetched” estimates that the NDI notification requirement will cost billions of dollars to industry. “These are things we are supposed to be doing already anyway,” he said, citing, for example, the need for testing or compiling safety literature on the ingredient.
Steve Mister, president and CEO of the Council for Responsible Nutrition (CRN), noted FDA has taken the position that companies should be preparing safety data “as a matter of business” before introducing a new supplement.
“When they do their cost analysis, FDA is looking at it from the standpoint of, ‘What will it cost you in addition to that [safety work] to fill out the paperwork and send it in to FDA to notify us?’” Mister said in a phone interview. “But the underlying work—the safety work—is not so much about compliance with NDIs, it’s doing business as a company that’s putting products on the market that people are going to swallow.”
Read more at naturalproductsinsider.com
Tagged Under: dietary supplements, FDA, government, NDI, supplements